Two FDA-approved small-molecule drugs, riluzole and edaravone, are currently available on the market; the antisense oligonucleotide tofersen was also recently approved. However, none blocks disease progression, making it crucial to investigate new therapeutic routes to overcome ALS.
Several products are known to slightly slow ALS progression, but they are not pursued by the pharmaceutical industry and academics because they lack patentability. One of the best-known among these products is TUDCA, while two lesser-known ones are Acetyl-L-Carnitine and Alpha-Lipoic Acid.
There are many forms of ALS, and each will likely require different therapeutics. Most forms are characterized by aggregates of misfolded TDP-43 fragments, an important protein. Various theories exist regarding how these aggregates form; one posits that they are a form of stress granules.
They screened many drug candidates and found that one, closely related to Alpha-Lipoic Acid, holds good prospects for future use as a drug. Indeed, it will likely be modified to enhance its bioavailability and thus could be patented.
The scientists screened a library of 1,600 small molecules to identify compounds that affect stress granule formation, using HeLa cells expressing FUS–GFP, a fusion protein that localizes to stress granules under stress. I remain cautious about findings from in vitro experiments, especially those involving immortalized cells of cervical cancer, whose biology often differs from that of normal cells. So consider yourselves warned, dear readers.
 The researchers specifically focused on molecules that could reduce or reverse stress granule formation, particularly those that act directly on stress granule proteins and may be useful as therapeutic agents. From the initial screening, lipoamide emerged as a novel, potent modulator of stress granules.
Once lipoamide was identified as a hit, the researchers sought to determine its effects in cells regarding specificity, potency, intracellular localization, and its effects on other cellular condensates.
The researchers specifically focused on molecules that could reduce or reverse stress granule formation, particularly those that act directly on stress granule proteins and may be useful as therapeutic agents. From the initial screening, lipoamide emerged as a novel, potent modulator of stress granules.
Once lipoamide was identified as a hit, the researchers sought to determine its effects in cells regarding specificity, potency, intracellular localization, and its effects on other cellular condensates.
It's beneficial to understand the underlying mechanism of action, even if it's often challenging to achieve in biology. Thus, the authors evaluated the structure–activity relationships (SAR). Understanding which chemical features are critical for its function will assist in enhancing the potency of a future drug. The authors thus aimed to clarify its mechanism of action—whether it alters the physical properties of condensates, binds to specific proteins, or acts via redox chemistry.
Lipoamide was found to prevent stress granule formation when added before stress and to dissolve existing stress granules when added afterward.
Lipoamide's effect was also specific to stress granules, without impacting other cellular condensates or general protein synthesis. This specificity is crucial as it minimizes the risk of adverse effects.
Proteins stabilized by lipoamide are enriched in intrinsically disordered regions (IDRs) with high arginine and tyrosine content—characteristics typical of stress granule proteins. These effects suggest that lipoamide interacts with these IDR-rich proteins to modulate condensate behavior, likely through nonenzymatic redox interactions.
In conclusion, the authors identified lipoamide as a promising small molecule that selectively and reversibly modulates stress granule formation. It does so not through conventional binding to a specific target, but likely via redox-sensitive interactions with disordered proteins, altering the physical properties of condensates.
This may have therapeutic implications for ALS and other neurodegenerative diseases linked to stress granule dysfunction. However, this research is not yet at the pre-clinical trial stage, which we know unfortunately provides limited information about success in human trials. This study is in vitro with cells that possess biology mostly alien to living beings. These cells are not motor neurons, so it's somewhat odd to see so many references to ALS in the text; it's probably a bait for investors.

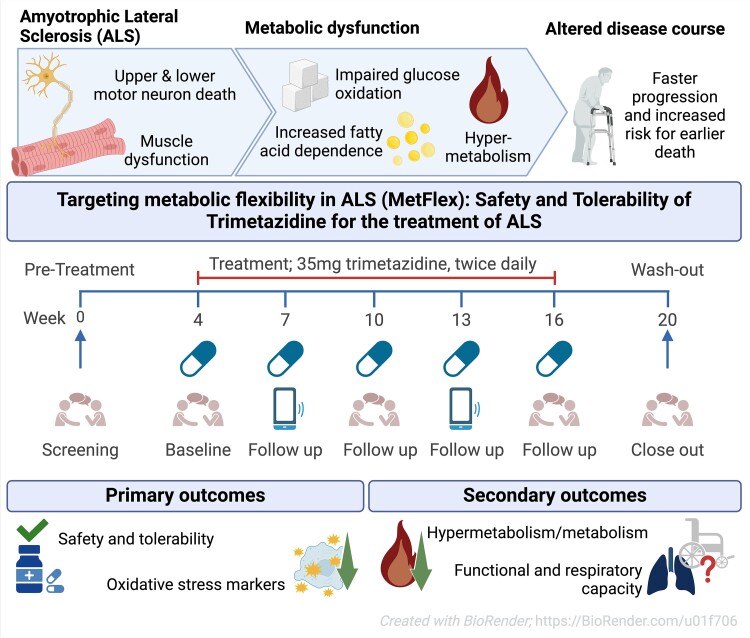 While the publication recounts that trimetazidine was beneficial for patients (this is not a phase III trial), for me the results section does not show conclusive results. For example, the results improved only during the wash-out period.
While the publication recounts that trimetazidine was beneficial for patients (this is not a phase III trial), for me the results section does not show conclusive results. For example, the results improved only during the wash-out period.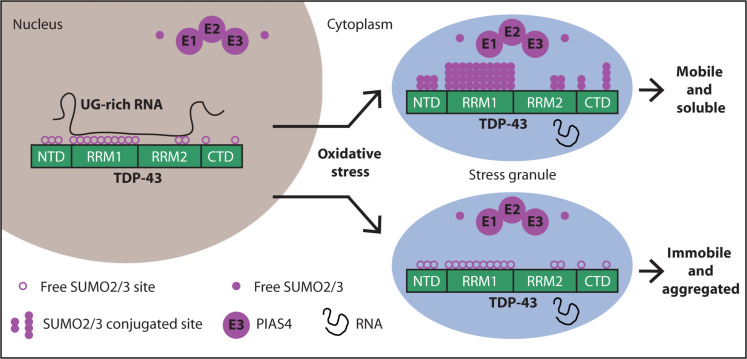 Transactive response DNA-binding protein 43 (TDP-43) is a nuclear RNA binding protein (RBP) involved in RNA metabolism.
TDP-43 has a high propensity to aggregate because of its low solubility in cells and in vitro.
The aggregation propensity of TDP-43 is increased by ALS/FTD-linked mutations and upon exposure to stress and has been observed in patients with C9orf72 hexanucleotide repeat expansion, the most common genetic cause of sporadic and familial.
Transactive response DNA-binding protein 43 (TDP-43) is a nuclear RNA binding protein (RBP) involved in RNA metabolism.
TDP-43 has a high propensity to aggregate because of its low solubility in cells and in vitro.
The aggregation propensity of TDP-43 is increased by ALS/FTD-linked mutations and upon exposure to stress and has been observed in patients with C9orf72 hexanucleotide repeat expansion, the most common genetic cause of sporadic and familial.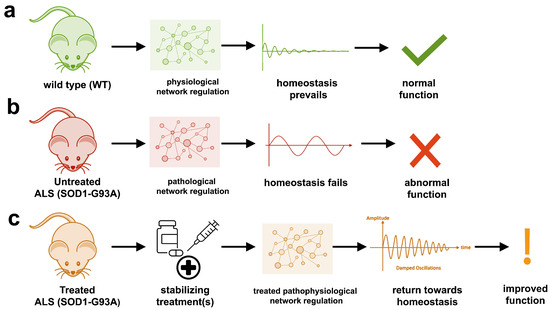 The study uses an innovative and integrative framework to model the regulatory dynamics of wild-type (WT) and SOD1-G93A ALS mice. The models are based on first-order ordinary differential equations (ODEs) that describe how the system output evolves over time. The research uses dynamic meta-analysis to synthesize experimental data from the literature and parameter optimization based on genetic algorithms to infer missing data. Indeed, to build a model, data are needed and here these are obtained from results reported in the literature on SOD1-G93A ALS mouse models.
The study uses an innovative and integrative framework to model the regulatory dynamics of wild-type (WT) and SOD1-G93A ALS mice. The models are based on first-order ordinary differential equations (ODEs) that describe how the system output evolves over time. The research uses dynamic meta-analysis to synthesize experimental data from the literature and parameter optimization based on genetic algorithms to infer missing data. Indeed, to build a model, data are needed and here these are obtained from results reported in the literature on SOD1-G93A ALS mouse models.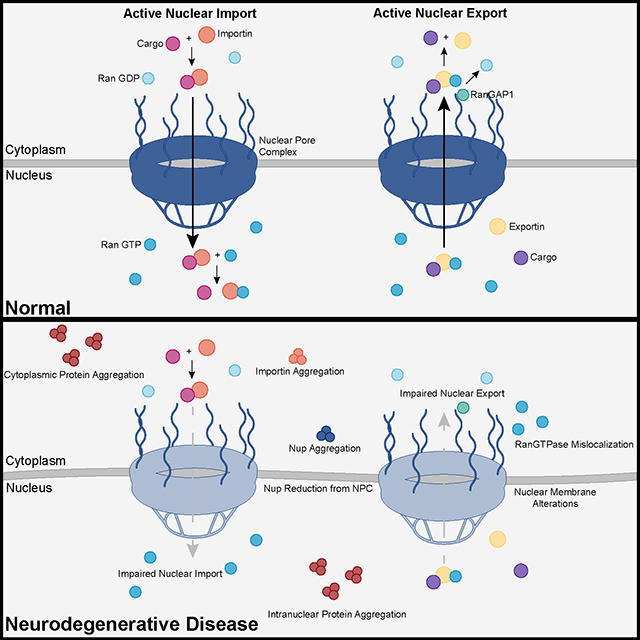 Recent advances into the underlying pathogenic mechanisms have associated mislocalization and aberrant accumulation of disease-related proteins with defective nucleocytoplasmic transport and its mediators called karyopherins.
Recent advances into the underlying pathogenic mechanisms have associated mislocalization and aberrant accumulation of disease-related proteins with defective nucleocytoplasmic transport and its mediators called karyopherins.  They measured somatic repeat expansion over time in individual neurons from donors of different ages. They found that early-phase expansions (e.g., from 40 to 80 CAG repeats) were slow and stochastic, taking decades, while later expansions (e.g., from 80 to 150 repeats) occurred more rapidly.
They then analyzed genetic markers and DNA repair mechanisms associated with repeat instability, such as those involving DNA mismatch repair (MMR) proteins (e.g., MSH3, PMS1). Variants in these genes have been shown to influence the rate of somatic instability. The progression of CAG repeat expansion is driven by errors in DNA replication, repair, and maintenance, particularly in neurons. Key mechanisms include:
They measured somatic repeat expansion over time in individual neurons from donors of different ages. They found that early-phase expansions (e.g., from 40 to 80 CAG repeats) were slow and stochastic, taking decades, while later expansions (e.g., from 80 to 150 repeats) occurred more rapidly.
They then analyzed genetic markers and DNA repair mechanisms associated with repeat instability, such as those involving DNA mismatch repair (MMR) proteins (e.g., MSH3, PMS1). Variants in these genes have been shown to influence the rate of somatic instability. The progression of CAG repeat expansion is driven by errors in DNA replication, repair, and maintenance, particularly in neurons. Key mechanisms include: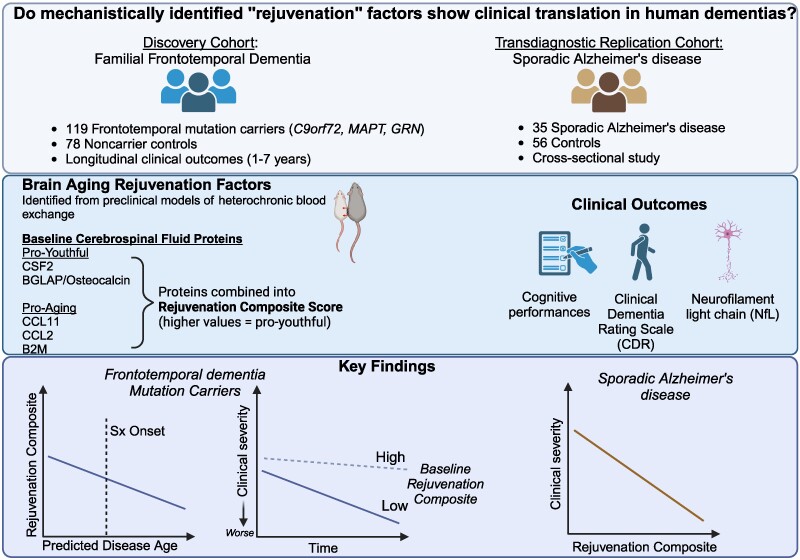 The results appear relatively reliable because the scientists found similar effects in two different types of dementia. The effects were seen across multiple measures (cognitive, functional, and biological markers).
The results appear relatively reliable because the scientists found similar effects in two different types of dementia. The effects were seen across multiple measures (cognitive, functional, and biological markers). The endoplasmic reticulum (ER) is an important organelle in cells that is involved in protein conformation. This step occurs after protein synthesis by ribosomes and after conformation, the new protein will be sent to its final destination by the Golgi apparatus. Protein conformation requires energy, so when disease occurs, the ER may not be able to properly conform the new proteins.
The endoplasmic reticulum (ER) is an important organelle in cells that is involved in protein conformation. This step occurs after protein synthesis by ribosomes and after conformation, the new protein will be sent to its final destination by the Golgi apparatus. Protein conformation requires energy, so when disease occurs, the ER may not be able to properly conform the new proteins. Several therapeutic strategies are in clinical development to restore PGRN levels in the CNS, including gene therapy. However, a limitation of therapeutic approaches aimed at alleviating pathologies associated with FTLD could be their ineffective diffusion across the blood-brain barrier.
Several therapeutic strategies are in clinical development to restore PGRN levels in the CNS, including gene therapy. However, a limitation of therapeutic approaches aimed at alleviating pathologies associated with FTLD could be their ineffective diffusion across the blood-brain barrier.